Clinical trial technology company Science 37 has raised $31 million in a series B round led by Redmile Group, with participation from Lux Capital, dRx Capital, and Sanofi-Genzyme BioVentures.
Founded out of Los Angeles in 2014, Science 37 is adopting a mobile-first approach to running clinical trials, as it strives to eradicate geographical barriers and make it easier to connect patients with scientists and researchers.
The problem Science 37 is working to fix is this: Clinical trials are traditionally set up around specific locales, or “sites,” and the patients are usually recruited from that area, which limits the pool trials can draw from. Science 37 offers what it calls “metasites,” which effectively brings the clinical trial to each patient’s home — they can continue to use their existing doctor, while also being offered telemedicine or home visits, if required. So any patient seeking to benefit from the latest cutting-edge treatments can do so without having to relocate or travel long distances, which cuts costs and time, while (in theory) accelerating research.
These metasites are underpinned by a cloud-based platform called NORA (Network Oriented Research Assistant), which helps centralize everything and joins the dots between patients, researchers, and health professionals.
June 5th: The AI Audit in NYC
Join us next week in NYC to engage with top executive leaders, delving into strategies for auditing AI models to ensure fairness, optimal performance, and ethical compliance across diverse organizations. Secure your attendance for this exclusive invite-only event.
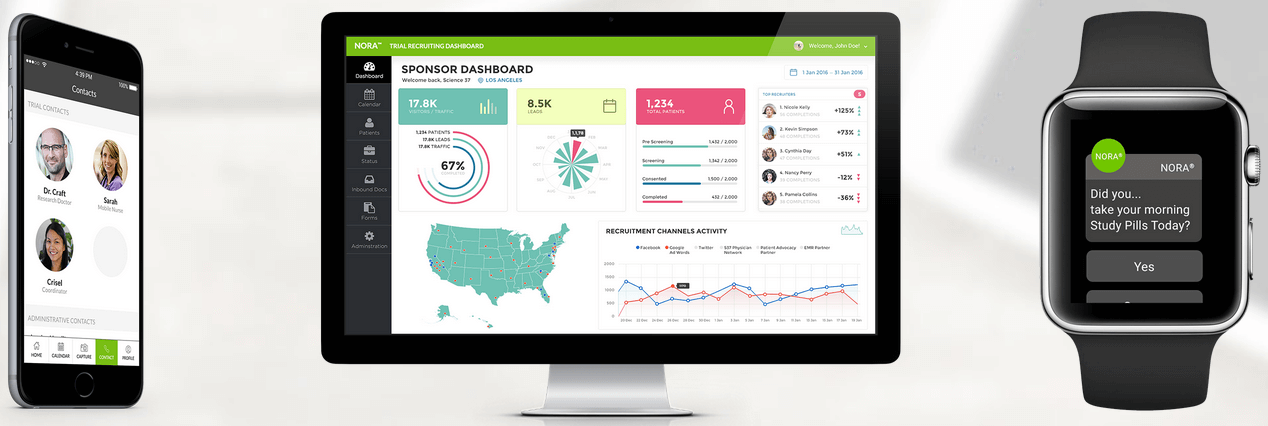
Above: Science 37: NORA
Prior to now, Science 37 had raised a $6.5 million funding round, and the company says that its latest cash influx will be used to scale its operations and expand access to its clinical trial technology.
“We started with the simple question, ‘What is best for patients?’ and built our technology and new clinical trial operating model around the answers,” explained Noah Craft, cofounder and CEO of Science 37.
With this latest series B funding, we will be able to create an even greater impact and achieve our mission of unlocking patient access to clinical trials. Our investors understand how we fit into the larger digital revolution that is forcing healthcare companies to think big, learn fast, adapt successfully, and enable patients to take a more proactive role in their own health. Our early successes last year demonstrated an exponential acceleration of trial speed that has led to incredible demand from biotech and pharmaceutical sponsors. We all share the common goal of bringing new cures to patients faster, and this investment will allow us to scale to meet the needs of our clients, while expanding into new therapeutic areas.
It’s also worth highlighting the role of Sanofi in the latest funding round. Sanofi is a billion-dollar multinational pharmaceutical company, and its investment extends beyond that of mere financial input — the strategic bet will allow Sanofi to provide “technical and strategic guidance” from its existing clinical trial resources around the world.


