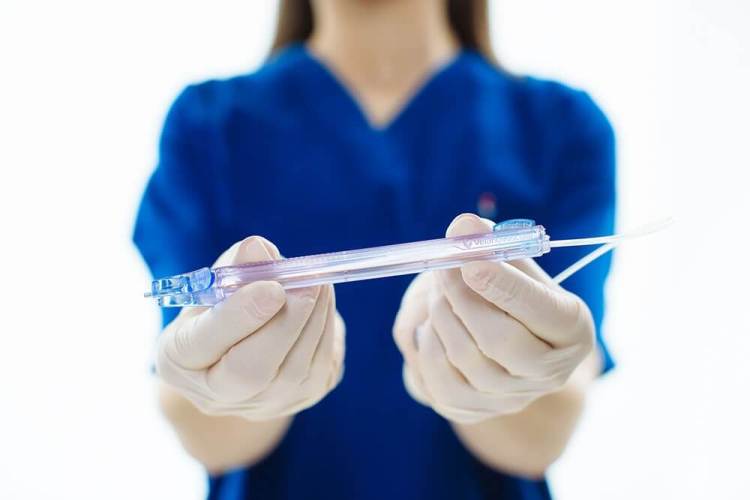
Blood drawing and testing may have been tainted by the Theranos scandal last year, but that hasn’t kept investors from backing Velano Vascular. The San Francisco-based startup, which has developed a needle-free device to draw blood, today announced a new investment of $17 million. This technology could reduce the risks of injuries for patients who suffer from difficult venous access due to weight, chronic disease, and other issues.
Velano Vascular’s product is called PIVO — a single-use, needle-free device that can be inserted through an existing (intravenous) IV catheter.
“Nearly all hospital patients already have an IV, a one-inch piece of plastic tubing, in their arm used predominantly for the administration of fluids and drugs,” wrote CEO Eric Stone, in an email to VentureBeat. “A peripheral IV catheter is quite effective at putting fluids into the body, but inconsistent according to most practitioners at taking blood out of the body. Our device turns an existing IV into a two-way conduit for drawing high quality blood samples.”
PIVO would help reduce the multiple needle sticks endured by hospitalized patients around the world. Stone says that such sticks are administered more than a billion times every year — in business terms, a sizeable market opportunity.
June 5th: The AI Audit in NYC
Join us next week in NYC to engage with top executive leaders, delving into strategies for auditing AI models to ensure fairness, optimal performance, and ethical compliance across diverse organizations. Secure your attendance for this exclusive invite-only event.
The chief executive would not disclose the price of the PIVO device, but he did say that Velano Vascular is currently working with health systems like Intermountain Healthcare, University Hospitals of Cleveland, Sutter Health, Children’s National Health System, Griffin Hospital, Brigham and Women’s Hospital, and others.
Velano Vascular recently announced its third FDA clearance — this time for the second generation of the PIVO device. “This latest design enables greater ease of use by practitioners and improved manufacturability so we can meet increased demand for the device,” wrote Stone.
This latest investment was led by two unnamed strategic investors, which Stone describes as a publicly traded global healthcare company and a supplier of a core component of Velano’s technologies. They were joined by existing investors Kapor Capital, First Round Capita, Safeguard Scientifics, and others. The new money will be used to ramp up manufacturing and sales of PIVO worldwide, as well as to develop additional vascular access technologies.
Founded in 2012, Velano Vascular has raised a total of $26 million to date and currently has approximately 12 employees.